Explain Different Types of Hydrogen Bond With Example
Hydrogen bonding in water. When hydrogen bonding takes place between different molecules of the same or different.

Hydrogen Bond Definition Types And Examples
The hydrogen bonds in the molecules of this man-made material are responsible for the crystallization of the material in the amide repeat unit.
. 2 Intramolecular hydrogen bond. Molecules are formed when atoms of either the same elements or different elements come together to share electrons and make covalent bondsThere are two types of attractive forces that keep the covalent molecules together. Acetylene has a triple bond a special type of covalent bond that will be.
The bond exits in the identical molecule. As an instance- hydrogen bonding in water and alcohol. In Hydrogen Fluoride HF the bond formed between H and F is a polar.
Nylon is used in many fabrics as well as other everyday items like toothbrushes and hairbrushes. Eq - HFHFHF H bonding. Hydrogen bonds form between neighboring water molecules when the hydrogen of one atom comes between the oxygen atoms of its own molecule and that of its neighbor.
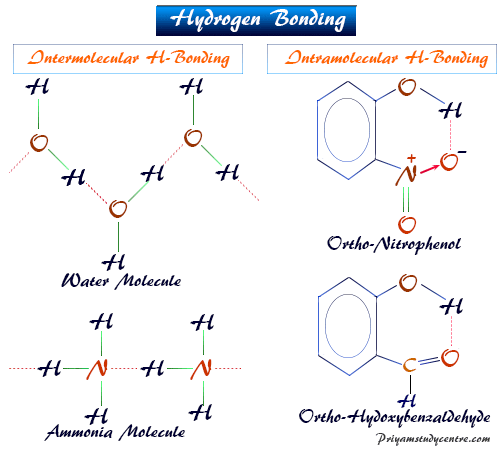
There are two types of H bonds and the classification of it is mentioned below 1Intermolecular Hydrogen bonding 2Intramolecular Hydrogen bonding 1Intermolecular Hydrogen bonding When hydrogen bonding takes place between different molecules of the same or different compounds it is called intermolecular hydrogen bonding. Hydrogen bonding in HF. Hydrogen bonding in water leads to the molecular association of.
Nylon a synthetic polymer famous for its stretchy qualities is another hydrogen bond example. Examples are water and alcohol. The hydrogen bonds in ammonia NH3 are formed between nitrogen and hydrogen atoms.
Main Difference Intermolecular vs Intramolecular Hydrogen Bonding. Intermolecular hydrogen bonding This type of bond formation takes place between the distinct molecules of equal or specific compounds. Depending on the specific type of bond and the particular species nature the bonds may be strong or weak.
This hydrogen bond is a special type in which the proton is usually placed in between the two identical atoms. Hydrogen bond formed between two different polar molecules of same or different substances is known as intermolecular hydrogen bond. Electrostatic force of attraction that exists between hydrogen atom of one molecule and electronegative atom of another molecule is called intermolecular hydrogen atom.
Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen. A covalent bond between a hydrogen and an atom of a strongly electronegative element is a polar covalent bond. Types of hydrogen bonds.
This is a. Bonds especially covalent bonds are often represented as lines between bonded atoms. Hydrogen Bonding Examples 1.
For this reason there is a partial positive charge on the hydrogen atom and a partial negative charge on the atom of the negative electronegative element. Depending on the occurrence of the hydrogen bonding hydrogen bonds are of two types. These molecules are associated together to form the cluster HF n.
The two strands of the famous double helix in DNA are held. The intramolecular hydrogen bond is the hydrogen bond formed between the hydrogen atoms and electronegative atoms like nitrogen oxygen and sulfur within the same molecule. Water ammonia and hydrogen fluoride are examples of such types of molecules.
The different chemical species like atoms ions and molecules exist together by bonds. Type of hydrogen bond. HF H20 NH3 etc.
1Intermolecular hydrogen bonding 2Intramolecular hydrogen bonding. Hydrogen bond forms due to the dipole-dipole interactive force of attraction. Nitrogen is a highly.
Alcohols phenols ester acids and amines are all examples of hydrogen bonding in organic chemistry. 2 Intermolecular Hydrogen bonding - It occurs among the different facts of same molecule. Fluorine is an.
Intramolecular hydrogen bonding This type of bond formation happens when the hydrogen atom lies in between the two electronegative elements gift inside the same molecule. 1 Intermolecular Hydrogen bonding - It occurs between two separate molecules. Types of Hydrogen Bonding Intermolecular Hydrogen Bonding.
Hydrogen bonding also occurs in organic molecules containing N-H groups. Hydrogen bonding in organic molecules containing nitrogen. Learn the definition some types and how hydrogen bonds are formed.
A chemical bond is a lasting attraction between two chemical species. Examples range from simple molecules like CH 3 NH 2 methylamine to large molecules like proteins and DNA. A hydrogen bond is formed between a hydrogen atom and a larger atom such as oxygen or nitrogen.
When there are hydrogen bonds between different types of molecules of the same or different types of compounds this is known as intermolecular hydrogen bonds. This happens because the hydrogen atom is attracted to both its own oxygen and other oxygen atoms that come close enough. Hydrogen bond formed between hydrogen atom of a molecule and highly electronegative atoms of the same molecule is known as intramolecular hydrogen bond.
In hydrogen fluoride HF the positive end of one dipole attracts the negative end of another similar dipole. This pictures shows examples of chemical bonding using Lewis dot notation. A water molecule is composed of a highly electronegative oxygen atom linked to the hydrogen atom.
These are two types of hydrogen bonds -. Hydrogen bond is a electrostatic attraction between a hydrogen atom which is bond to a more electronegative atom such as Nitrogen Oxygen fluorine. When the hydrogen bond is formed in between more than one molecule.
These are two types of hydrogen bonds - 1 Intermolecular Hydrogen bonding - It occurs between two separate molecules. Water ammonia hydrogen fluoride are all examples of hydrogen bonding in inorganic chemistry. The hydrogen bonding which takes place within a molecule itself is called.
Recall the hydrogen bonds that occur with ammonia. Hydrogen bonding in water alcohol.

Hydrogen Bond Definition Types And Examples

No comments for "Explain Different Types of Hydrogen Bond With Example"
Post a Comment